Submitted by L.M. Elmer on Mon, 14/09/2020 - 12:33

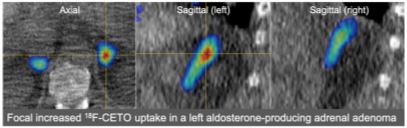
The first clinical study involving a First-Time-in-Human trial of an Investigational Medicinal Product performed under the auspice of the Cambridge Clinical Trials Unit has recently been initiated by a project team led by Franklin Aigbirhio (WBIC) and Mark Gurnell (Institute of Metabolic Science) and researchers from Radiology (Fiona Gilbert and Luigi Aloj), Mathematical Statistics (Sergio Bacallado) and Queen Mary London (Morris Brown). Funded by an MRC Developmental Pathway Funding Scheme award, the objective of the trial is to develop an improved diagnostic technique to localise the site(s) of autonomous aldosterone production in primary aldosteronism (PA), a common and potentially curable form of hypertension.
The new technique involves positron emission tomography (PET) coupled with a new radiopharmaceutical which selectively binds to the enzyme aldosterone synthase (highly expressed in aldosterone-producing adrenal adenomas) and is being produced at the WBIC Radiopharmaceutical Unit led by Istvan Boros. The first two studies supervised by the project’s Clinical Fellow Russell Senanayake have progressed well, laying the platform to perform further studies over the next year and towards the project’s overarching aim of establishing this as a new NHS diagnostic technique.